
H. Pylori Urea Breath Test Supplier | Headway
Founded in 1996, Headway is a supplier of urea breath test kits and analyzers for Helicobacter pylori. Headway offers urea [¹³C] capsule breath test kits, urea [¹⁴C] breath test kits, ¹⁴C Helicobacter pylori urea breath analyzers, and ¹³C urea breath test detectors.
Carbon13 Urea Breath Test Kit & UBT Detector
Headway provides C13 urea breath test kits and UBT detectors(HCBT-01). The test process is highly safe, painless, non-invasive, and non-radioactive. It is suitable for a wide range of people, including pregnant women and children.
Carbon14 Urea Breath Test Kit & UBT Analyzer
Headway offers C14 urea capsule breath test kits and UBT analyzers(HUBT-20P). The test process is highly safe, painless, and non-invasive, and the test results are simple, fast, accurate, and sensitive.
News & Blog
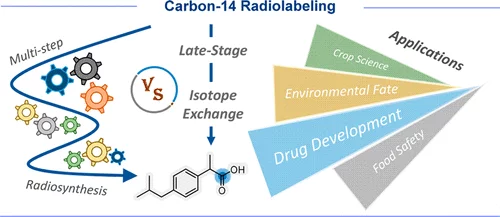
14C Radiolabeling: Technical Analysis and Customization Service Guide
Explore the comprehensive guide to 14C radiolabeling technology and customization services. Headway offers professional custom 14C radiolabeling compound services to meet complex research and regulatory requirements.

Helicobacter Pylori Treatment Guidelines: Symptoms, Risks & Solutions
Discover comprehensive insights into Helicobacter pylori treatment guidelines: infection symptoms, risks, diagnosis, and treatment. Embrace Headway’s accurate 13C and 14C Urea Breath Testing and scientific eradication solutions.
Medica 2024 is coming
From November.11 to 14, 2024, the Medica 2024 will be held in Messe Düsseldorf, Germany and Headway will send a team of staff with the company’s breath test products to participate in the exhibition. MEDICA
DDW 2024 is coming
From May.18 to 21, 2024, the Digestive Disease Week (DDW 2024) will be held in Washington D.C. USA and CNNC Headway will send a team of staff with the company’s breath test products to participate
Qualification Certificates
About Us
About Us
For over 20 years, Headway has established 3 product research and development centers, including the Pharmaceutical R&D Department, the Equipment R&D Department, and the Isotope R&D Department. With carbon-13 and carbon-14 breath diagnostics as the core technology, the Headway R&D Center continuously upgrades Helicobacter pylori detection technology, improving the accuracy of Helicobacter pylori testing. Our product quality has passed international quality certifications such as ISO 9001, ISO 13485, and TUV ISO13485, making us a trusted manufacturer of urea breath test products.
Headway’s factory covers an area of over 7,000 square meters, with multiple production lines and a monthly output of 3.25 million. Our manufacturing center includes the Pharmaceutical Production Department, Process Engineering Department, and Material Storage and Transportation Department, featuring advanced production and testing processes for Helicobacter pylori urea breath test kits.
Our UBT test products are exported to over 74 countries and regions, and we currently collaborate with more than 2,000 medical institutions, our client base includes medical representatives, physicians in public and private hospitals, clinical research professors, members of the National Gastroenterology Committee, and other medical clients.We provide wholesale service of UBT test kits & analyzers technical service support worldwide, offering products and services to global partners. Headway has become a world-leading company in urea breath test products.
User Experience


FAQs
Headway has established a quality management system for Helicobacter pylori urea testing instruments. In 2015, Headway passed the ISO 9001 quality management system certification.
Headway continuously designs and develops urea breath test devices by requirements, ensuring they are safe for diagnosing use. In 2016, we passed the ISO 13485 quality management system certification and the TUV ISO 13485 quality management system certification.
Headway supplies C13 urea breath test devices, C13 urea breath analyzers that support 6-channel batch testing, C14 urea breath test kits, and C14 urea breath test detectors. Click on the product page to quickly view our product list. We will continue to develop new HP breath analyzer products in the future, and new products will be updated in the product list.
Our product packaging includes a complete instruction manual for users’ reference. In addition, Headway has set up sales, after-sales, and technical support teams to provide customers services to solve relating problems.
Headway is the first manufacturer of Helicobacter pylori urea testing tools to produce C-14 using a commercial reactor. In the past, Headway used small research reactors to produce carbon-14, but intermittent production led to small yields and an unstable supply. Commercial nuclear power units can operate at high power stably for long periods. Headway has experimented with using commercial nuclear reactors to produce C-14, achieving continuous and stable production of carbon-14. Headway uses C-14 to make capsules and produces C-14 breath testing tools for diagnosing Helicobacter pylori infections.



