On March 1 2024, a new urea [¹³C] breath test kit developed by Shenzhen Headway Biotechnology Co., LTD. successfully obtained the drug clinical trial approval notice approved by the China Medicine Administration. Headway is the first similar [¹³C] Urea test capsule to declare and obtain the clinical trial approval notice in China.
Helicobacter pylori (HP) is a spiral-shaped gram-negative bacterium with unipolar sheathed flagellae often tipped with a distinctive bulb. 
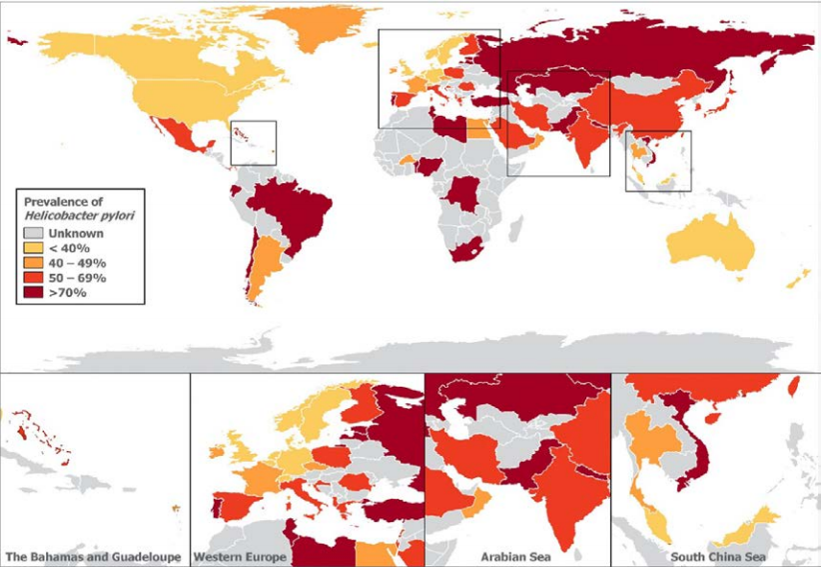
One third of adults are infected in northern Europe and America, more than half are infected in south and east Europe, South America, and Asia, and over two-thirds of Africans. It follows that HP prevalence is higher in first and second generation immigrants from developing to developed nations.
With an Innovative development path driven by R&D and innovation, CNNC Headway further enhances its core competitiveness by continuously developing new drugs, making it easier for human beings to pursue health.