As one of the world’s largest suppliers of custom carbon-14 radiochemicals, Headway has an excellence reputation in synthetic chemistry enabling our customers to carry out the most challenging synthetic projects.
Headway has a global customer base encompassing both the Medical sciences and chemical industries, such as the pharmaceutical development, clinical trial, human health, animal health, crop protection, food and nutrition, household, pesticides development and environment monitoring.
We provide comprehensive Good Manufacturing Practice (GMP) synthesis services for 14C-labeled compounds tailored for human studies. Customers who entrust both their preclinical and clinical research projects to us benefit significantly. By avoiding the necessity of re-synthesis, they can expedite their journey to clinical trials and reduce development costs substantially. Headway R&D cycle typically spans 2-3 months. We take immense pride in always ensuring timely and complete delivery to meet our customers’ expectations.
Figure 1: Metabolic pathway analysis using 14C-labeled compounds
To establish potential issues affecting human health and is required worldwide by regulatory agencies such as OECD, FDA, and EMA for the following:
(1) pharmaceutical development: to unveil drug metabolism, disposition, and pharmacokinetics of novel pharmaceuticals;
(2) animal health drug development: to determine the metabolism, disposition, and pharmacokinetics of veterinary drugs;
(3) crop science: to understand the plant metabolism of agrochemicals and pesticides;
(4) human food safety evaluation: to ensure that food derived from animals that have been treated by veterinary drugs is safe for human consumption;
(5) environmental fate studies: to perform soil dissipation studies and assess potential environmental impacts associated with human and animal health product excretions that might enter the aquatic and terrestrial environment.
Authoritative clinical trial guidelines highlight the necessity of radiolabeling compound
Whether you require a synthesis or repurification under GMP, we perform both in compliance with EMEA and the recent FDA Phase I GMP guidance (IQCH Q7A Section 19: Single batches for investigational drugs).
Radiolabels shall be positioned at sites (one or more as necessary), to facilitate elucidation of metabolic and transformation pathways and to facilitate investigation of the distribution of the active substance and of its metabolites, reaction and breakdown products (EU 283/2013).
Data on metabolism together with a schematic diagram of the metabolic pathways in plants and animals are required and these studies shall be conducted with one or more radio-labelled forms of the active substance and, where relevant, stereoisomer forms of the active substance and its metabolites (EU 283/2013).
Reasons for choosing carbon-14
- 14C is the regulatory isotope of choice
- It has a long half-life, thus no requirement to correct for decay in extended studies
- Defined label position(s) in the core structure of the molecule
- Compounds can be prepared using tailored custom synthesis
- Provides a highly sensitive method for Detection which is Quantitative & Qualitative
- Allows fate of parent and metabolites to be followed and pathways elucidated
Why Go for Headway
1.A short-lived development phase
The R & D cycle at Headway typically extends over a period of 2 – 3 months. We are extremely proud of our unwavering commitment to ensuring punctual and comprehensive delivery, consistently meeting and even exceeding our customers’ expectations.
2.Leading 14C-GMP manufacturing
Headway offers all-inclusive Good Manufacturing Practice (GMP) as well as Good Laboratory Practice (GLP) synthesis services for 14C-labeled compounds that are custom – designed for human studies.
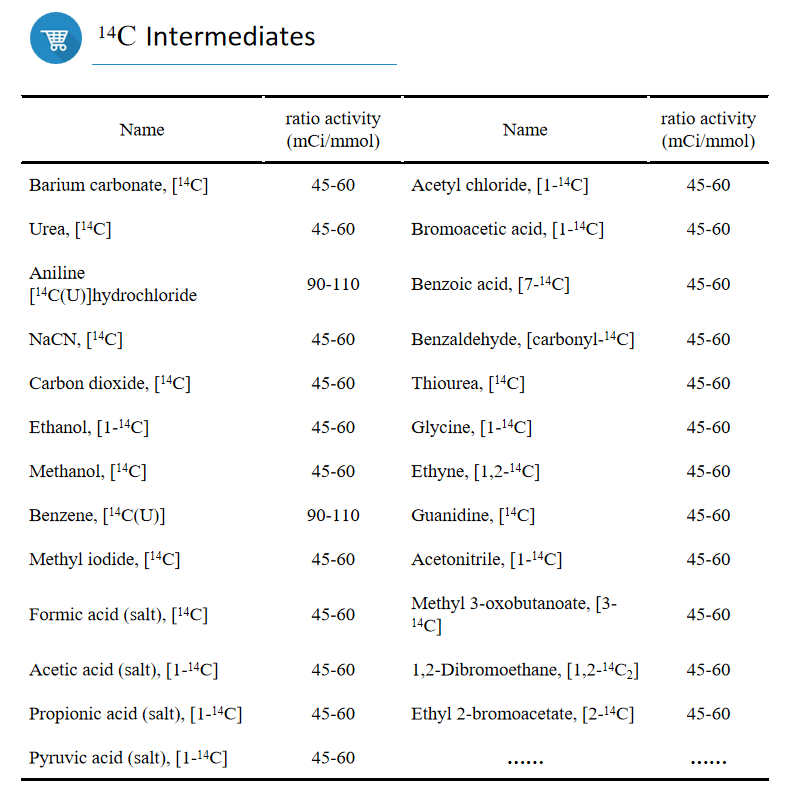
3. 5 product categories and 60+ intermediates
Headway offers an all-encompassing service, which encompasses synthesis, repurification and long-term storage.
4.Well-equipped analytical laboratories
Headway has 3,000 m2 dedicated testing site and an impressive array of instrumentation.
Contact Us:
Asia Pacific Sales Manager:Meirong Tang +86 13602679858
Latin American Sales Manager:Nana Liu +86 18617050092
International Sales Director: Felix Zhang +86 15807077009





