The Urea breath test kit is specifically designed to detect Helicobacter pylori (H. pylori) infection. This bacterium is linked to many gastrointestinal diseases, such as peptic ulcers and stomach cancer.
The kit provides a convenient and accurate way to conduct a diagnosis. Its user-friendly design requires minimal training, allowing all users to perform the test with confidence and precision.
Moreover, the H.pylori breath test kit offers a non-invasive alternative to conventional diagnostic procedures. Unlike endoscopy, it does not require anesthesia. This makes the testing process more comfortable for patients.
Table of Contents
ToggleWhat are Urea Breath Test Kits?
The Urea breath test kit has a universal application in diagnosing H. pylori infections. Both 13C-UBT and 14C-UBT methods are widely used. The following key advantages make these UBT kits more effective and accessible.
(1) Advantages of Urea Breath Test Kit
- High sensitivity and specificity
- Non-invasive
- Deliver quick results within 5to 10 minutes
- Can be used in diverse environments
- Safe
(2) Components of the UBT Kit
The breath test kit for H.pylori includes several essential elements that facilitate the diagnosis:
- Urea Capsule
This is a special capsule that contains urea labeled with radioactive isotopes, such as carbon-13 or carbon-14. If H.pylori is present in the stomach, it breaks down the urea. This process releases carbon-13 or carbon-14 dioxide that can be detected in the breath.
- Breath Collection Device
This device collects breath samples from the patients after they ingest a urea capsule. It usually comprises air bag or cards that capture and store exhaled air for later analysis.
- Analyzing Equipment
This equipment measures the carbon dioxide level in the breath samples. Techniques like six-channel detection can be used to detect labeled carbon-13 dioxide, which indicates an active H.pylori infection.
(3) Types of UBT Kits (13C and 14C)
A Urea breath test kit is mainly classified into two types based on the isotope used: 13C-UBT kits and 14C-UBT kits.
- 13C–UBT kits
This kit uses carbon-13, a stable and non-radioactive carbon isotope. It is safe for children and pregnant women. Its non-radioactive nature allows for repeated testing without concerns about radiation exposure.
- 14C–UBT kits
This type of urea breath test kit utilizes carbon-14, a limited radioactive isotope. The radioactive component requires strict regulations for use and disposal. Safety concerns restrict its use in certain populations. It also got U.S. FDA and China FDA exemption.
The Role of Urea Breath Test Kit in H. pylori Detection
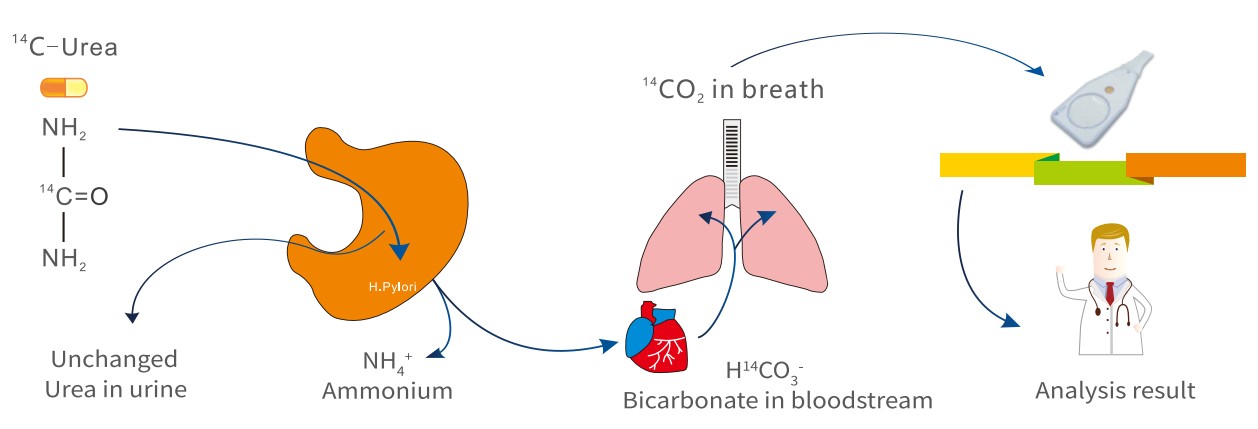
A Urea breath test kit plays an important role in detecting H.pylori infections by providing a non-invasive and rapid diagnostic method. It works by measuring labeled carbon dioxide in breath after the patient ingests a urea capsule.
Principle of Detecting Labeled Carbon:
The detection of labeled carbon in breath depends on the H. pylori’s metabolic activity. H. pylori contains the urease; the urease breaks down urea. It releases CO2 with a 13C or 14C isotope from the ingested capsule.
The urea breath test machine measures the labeled CO2 concentration in your breath. The higher levels of labeled carbon dioxide concentration confirm an active H.pylori infection.
A Urea breath test kit is a preferred non-invasive choice for detecting H.pylori before and after treatment. It reduces patient anxiety associated with medical testing and improves comfort. It also increases clinical efficiency with streamlined processes and flexibility for use in clinics or at home.
Detailed Procedure of Urea Breath Test Kit
Here’s a step-by-step guide to conducting H.pylori diagnostic test using the UBT kit:
1. Preparation
Make sure the patient fast for at least 2 hours. Avoid certain medications, such as proton pump inhibitors (PPIs) and antibiotics for 2 weeks.
2. Taking A Capsule
The subject should swallow one urea(¹³C or 14C) capsule with cool water and wait a specific period of time.
3. Sample Collection
Collect baseline and post-ingestion breath samples as instructed on the urea breath test kit.
4. Testing
Once the samples have been collected, insert them into the urea breath test analyzer for measurement and analyse.
The Supply of Urea Breath Test Kits from Headway
Headway is a trusted supplier of urea breath test kits with ISO 9001, ISO 13485, and GMP certifications. For high quality, reliability, excellent support, and ease of use in 13C and 14C urea breath test kits, consider entrusting your requirements to Headway.
Advantages & Applications of UBT Kits by Headway:
1. 13C Capsule Breath Test Kit
Here are some key benefits and applications of this kit reading its use:
Advantages
- Safe to use
- Non-invasive
- Quick results
- Non-radioactive
- Painless
- High sensitivity and specificity
Applications
- Use in various clinical and research settings
- Use in post-monitoring treatment to confirm eradication of H.pylori infection
2. 14C Urea Breath Test Kit
The H.pylori 14C urea breath test kit by Headway is useful for:
Advantages
- The radiation level is low and poses no harm
- Non-invasive
- Fast results
- Highly precise
- Convenient and handy
Applications
- Initial diagnosis of dyspepsia
- Unexplained iron deficiency anemia
- Long-term use of NSAIDs
- Chronic active gastritis
- Refusal of gastroscopy
- Idiopathic thrombocytopenic purpura (ITP)
Conclusion
The urea breath test kit is a non-invasive procedure to detect H. pylori infection. Both 13C and 14C UBT kits are highly accurate. They require only the ingestion of a urea capsule followed by breath analysis.
The ability of UBT kits to monitor treatment effectively makes them invaluable diagnostic tools. Hospitals should consider employing these kits from Headway due to their proven reliability and ease of use.